
A few days ago, I learnt about the three states of matter which are Solids, Liquid and Gases!, and what the particles (molecules) do, the definition of chemical changes, what are atoms/learning the Periodic Table and many more!
Liquid particles are together like a solid, but maneuvers around. (The bonds in the liquid is strong enough to keep the particles together, but also weak enough to let them flow together, kind of like a solid).
Solid particles are intact and stays together! (The molecules in a solid vibrates, but do not move past together like a gas. The solids retains its shape or mould).
Gas particles like to be separated! The molecules in gases move rapidly and past other molecules!
The definition of "Chemical Changes" is when a substance is combined with another substance to create a new type of substance. A chemical change is irrevocable, which has to include the rearrangement of either atoms or, 'as mentioned above' when two or more substances are mixed to create a new substance. An example of a chemical change is wood being burned, ever so slightly that ashes start to form and parts of the wood will turn color (pitch black) and eventually burn, that is a chemical change, it cannot been turned to it's original form unlike a "Physical Change", which in this case can be turned back.


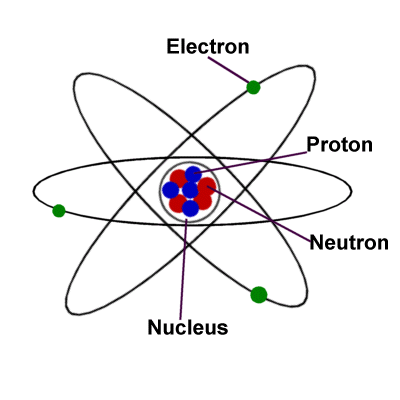
Moving onto Atoms, What are Atoms, you might be dying to hear about?, well an atom is a “particle” that can only be seen using a machine (microscopic machine). An atom is also indivisible, which means, however many times you cut an object or thing etc, it cannot be divided! There is also 3 other things that are involved which are Protons, Neutrons and Electrons.
What is a proton? A proton is a particle apart of Atoms, that is positively charged.
What is a neutron? A Neutron is also another particle of atoms and in which are neutral.
What is an electron? A electron is like a proton, accepts that they are both attracted to each other, but an electron has no electrical charge.

Above is the Periodic table!
0 comments:
Post a Comment
Note: only a member of this blog may post a comment.